Have you ever picked up a prescription and noticed the pill looks exactly like your brand-name drug-but the box says something completely different? Maybe it’s labeled with just the chemical name, no fancy logo, and costs significantly less. That’s not a mistake. It’s an authorized generic.
Most people think generics are cheaper versions made by different companies. But authorized generics are different. They’re made by the same company that made the brand-name drug. Same factory. Same ingredients. Same quality control. The only real difference? No brand name on the label.
How Authorized Generics Are Different From Regular Generics
Regular generic drugs must prove they work the same way as the brand-name version. That means they go through a process called the Abbreviated New Drug Application (ANDA). They have to show they’re bioequivalent-meaning your body absorbs them the same way. But they don’t have to use the exact same inactive ingredients. That’s why a generic version of your pill might be a different color, shape, or size. Sometimes, people notice the change and worry it’s not the same.
Authorized generics skip that step. They’re not new drugs. They’re the exact same product the brand-name company already made. Same active ingredient. Same inactive ingredients. Same coating. Same manufacturing line. The only thing that changes is the packaging and the name. The FDA calls them “exactly the same” as the brand-name drug. That’s not marketing speak-it’s the official definition.
And here’s the kicker: authorized generics aren’t listed in the FDA’s Orange Book, where all regular generics are published. That’s because they don’t go through the ANDA process. They’re covered under the original brand’s New Drug Application (NDA). So if you’re looking for them in the Orange Book, you won’t find them. You have to check the FDA’s separate list of authorized generics.
Why Do Brand-Name Companies Make Their Own Generics?
It sounds strange, right? Why would a company that spent millions developing a drug and making it profitable suddenly sell a cheaper version of it? The answer is strategy.
When a brand-name drug’s patent runs out, other companies can legally make copies. But those copies take time to get approved. The first generic maker often gets 180 days of exclusive rights to sell. That’s a big window to grab market share. So what do brand companies do? They launch their own generic version-right before or right during that 180-day window.
By doing this, they capture a chunk of the market before other generics even arrive. And because it’s the exact same drug, pharmacies and patients who trust the brand are more likely to switch to the cheaper version-especially if it’s priced lower than the original but still higher than the competition. This keeps revenue flowing while still offering savings.
Between 2010 and 2019, there were 854 authorized generic launches in the U.S. The biggest spike came in 2014. That’s not random. It’s a calculated move. In 75% of cases, the brand company waited until a regular generic had already entered the market before launching their version. That’s not about helping patients-it’s about protecting profits.
What Does an Authorized Generic Look Like?
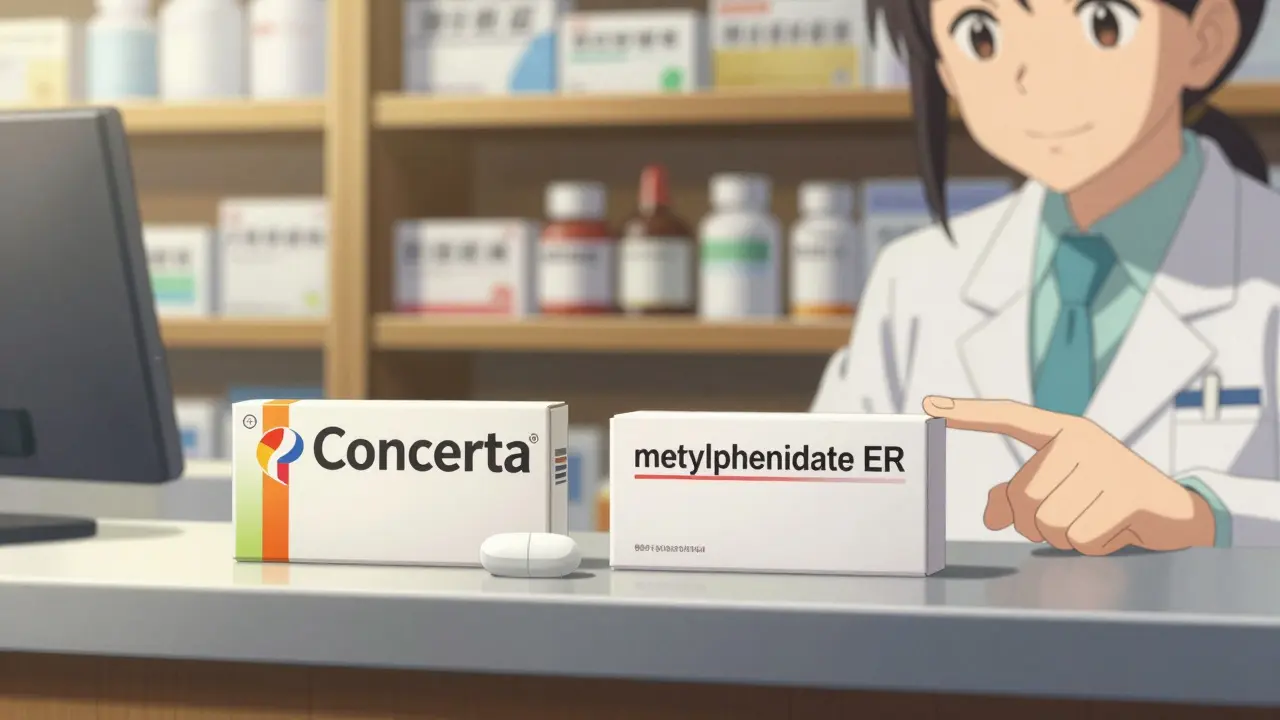
Visually, it’s almost identical to the brand-name drug. Same size. Same shape. Same color. Same imprint. The only thing different is the label. Instead of “Concerta,” you’ll see “methylphenidate ER.” Instead of “Celebrex,” you’ll see “celecoxib.”
Some authorized generics are made by the brand company itself. Others are made by a partner company under license. For example:
- Prasco Laboratories makes the authorized generic of Colcrys (colchicine)
- Greenstone Pharmaceuticals makes the authorized generic of Celebrex (celecoxib)
- Watson/Actavis made the authorized generic of Concerta (methylphenidate ER)
These aren’t knockoffs. They’re the real thing, just without the brand name.

How Much Do Authorized Generics Cost?
They’re cheaper than the brand-name drug-usually 15% to 25% lower. But they’re often more expensive than regular generics, especially once multiple generic makers enter the market. Why? Because they’re still tied to the brand’s pricing structure.
For example, if the brand-name drug costs $150 for a 30-day supply, the authorized generic might be $110. But after a few other generics arrive, the price could drop to $30. So timing matters. If you get the authorized generic early, you save money. If you wait, you might save even more.
Some patients report savings of 20% to 30% compared to the brand. But that’s not guaranteed. It depends on the drug, the pharmacy, and the insurance plan.
Are Authorized Generics Safe?
Yes. Absolutely.
They’re made under the same FDA inspections as the brand-name drug. Same equipment. Same quality checks. Same batch testing. There’s no risk of lower quality. In fact, because they’re identical in every way-including inactive ingredients-they’re less likely to cause unexpected reactions in people sensitive to fillers or dyes.
Some patients have reported side effects when switching to regular generics because of different inactive ingredients. That’s rare, but it happens. With authorized generics, that risk disappears. If your body tolerated the brand-name drug, it will tolerate the authorized generic without issue.
Why Are Authorized Generics Confusing?
Because they’re not labeled as generics. Pharmacists don’t always explain them. Insurance companies don’t always track them separately. Patients get a pill that looks like their old one, but the price is lower-and they don’t know why.
Some people think it’s a trick. “Why is my doctor giving me a ‘generic’ of my own brand?” Others worry it’s counterfeit. Pharmacists have to spend extra time explaining that this isn’t a cheaper version-it’s the same thing, just sold under a different name.
It’s also hard to know when an authorized generic is available. Unlike regular generics, they’re not in the Orange Book. You have to check the FDA’s authorized generic list or ask your pharmacist directly. Many don’t even know it exists.

How to Spot an Authorized Generic
If you’re curious whether your prescription is an authorized generic, here’s how to find out:
- Check the label. Does it have the brand name? If not, ask your pharmacist.
- Look up the drug name on the FDA’s List of Authorized Generic Drugs (updated October 10, 2025).
- Ask your pharmacist: “Is this the authorized generic version?”
- Compare the pill imprint and color to your previous brand-name version. If it’s identical, it’s likely an authorized generic.
Pharmacists can usually tell you right away. If they’re unsure, they can call the manufacturer or check the FDA’s list.
What This Means for You
If you’re paying full price for a brand-name drug, ask if an authorized generic is available. It’s not always cheaper than the competition, but it’s safer than a regular generic if you’ve had issues with inactive ingredients in the past.
It’s also worth asking if your insurance covers it. Sometimes, insurers push regular generics because they’re cheaper. But if you’ve had bad reactions before, or you just want the exact same drug, the authorized generic is the closest thing to the brand without the brand price tag.
Don’t assume all generics are the same. Authorized generics are a middle ground-offering the reliability of the brand with the cost savings of a generic. And if you’re someone who notices every little change in your meds, this might be the best option you didn’t know about.
What’s Next for Authorized Generics?
They’re not going away. As more brand-name drugs lose patents, companies will keep using authorized generics as a tool to hold onto market share. Regulators are watching. Some experts worry this practice slows down real competition by blocking cheaper generics from gaining ground.
But for patients? It’s not all bad. It’s another option. One that’s safe, reliable, and sometimes, just what you need.
If you’re on a medication that’s about to go generic, ask your doctor or pharmacist: “Is there an authorized generic?” You might be surprised by the answer-and the savings.

Comments
This is exactly why I stopped trusting pharmacies. They hand you a pill that looks identical but act like it's some kind of miracle discount. No explanation, no warning. Just 'here, take this.' I found out mine was an authorized generic only because I compared the imprint with my old bottle. No one bothered to tell me.
I never knew this existed. My mom takes blood pressure medicine and she said last month her pill changed color. She was scared. Now I understand. Same medicine, different box. Good to know.
Let’s be real - this isn’t about patient safety. It’s corporate strategy disguised as a cost-saving option. Brand companies don’t launch authorized generics to help you. They do it to choke out real competition before it even gets off the ground. They know patients are loyal to the look and feel of their meds. So they make a copy, slap on a plain label, price it just low enough to feel like a deal, and watch the real generics get buried under the weight of their own product. It’s predatory capitalism with a white coat. And the FDA? They’re fine with it because it’s technically legal. But don’t tell me this is patient-centered care. It’s market manipulation dressed up as transparency.
The FDA’s Orange Book omission is a regulatory loophole of monumental proportion. Authorized generics circumvent ANDA requirements under the NDA umbrella, which fundamentally undermines the statutory intent of the Hatch-Waxman Act. This isn’t 'same drug, different label' - it’s a legal fiction engineered to delay generic market saturation. The 75% statistic cited? That’s not coincidence. It’s collusion by proxy. And the fact that pharmacists aren’t mandated to disclose this? That’s malpractice by omission.
I used to panic every time my pill looked different. Then I learned about authorized generics. Now I ask my pharmacist every time I refill. If it looks the same but costs less? I grab it. No stress. No guesswork. And honestly? I’ve never had a problem with one. If you’ve had bad reactions to generics before, this is the quiet hero you didn’t know you needed.
The pharmaceutical-industrial complex has perfected the art of deception under the guise of consumer benefit. Authorized generics are not an innovation - they are a calculated erosion of market competition, enabled by regulatory capture. The FDA’s complicity in permitting this practice is a betrayal of public trust. One must question: who truly benefits? The patient? Or the shareholder?
There is a profound irony here: the same system that celebrates innovation and intellectual property now weaponizes its own creations to suppress the very competition it was designed to encourage. The authorized generic is not a gift to the consumer - it is the ghost of monopoly haunting the marketplace. We have replaced true choice with the illusion of choice. And in doing so, we have turned healthcare into a theater of economics, where the patient is both audience and actor - but never the author.
I work in a pharmacy and I can tell you - patients are shocked when they find out their brand-name drug is actually the same pill with a plain label. We have to explain it over and over. But once they get it? They’re relieved. No more anxiety about 'is this the same?' Especially for people with allergies to fillers - this is the safest option out there. Stop treating it like a scam. It’s the closest thing to the brand without the markup. And yeah, it’s totally okay to feel weird about it at first. But this isn’t shady. It’s smart.
The authorized generic is a mirror held up to the hypocrisy of modern medicine. We celebrate the idea of generic drugs as democratic access - yet we allow the original innovator to own the exact same product and sell it cheaper, just to keep the market from breaking. It’s not about health. It’s about control. We’ve turned medicine into a commodity with emotional branding, and now we’re surprised people don’t trust the system. Maybe we should stop pretending the system is benevolent.
I’ve been on the same medication for 12 years and I never knew this was a thing until my pharmacist mentioned it last week. I was so worried I’d have side effects, but I switched and felt exactly the same - honestly, better because it cost half. I told my mom and she’s switching too. If you’ve ever been scared to switch meds, please, ask your pharmacist about authorized generics. They’re not scary. They’re just the same pill without the fancy packaging. And honestly? You’re not losing anything. You’re gaining peace of mind and savings. It’s a win-win if you know to ask.
This whole system is a scam. Companies are allowed to make their own generics to manipulate the market? That’s not innovation - that’s fraud. And now patients are supposed to be grateful? No. If you’re going to make a copy, let someone else do it. Don’t pretend you’re helping people while you’re hoarding profits. This isn’t healthcare. It’s corporate theater.